An introduction to Enzymes – specific catalysts.
- Most are proteins.
- Few RNAs have enzymatic activity.
- Catalyze biochemical reactions.
- Increase the rate of reaction.
- Act repeatedly.
- Needed in small quantities.
- Substrate specific.
- Normally aqueous medium is required.
- Have high molecular weights.
- Catalytic activity is lost when they are denatured or dissociated in to subunits.
- Some enzymes do not need any chemical group other than its amino acid residues, but others require an additional chemical component which is known as cofactor or coenzyme.
- Enzymes are classified by the reactions they catalyzed.
| Type of reaction catalyzed | Class |
| Transfer of electrons | Oxidoreductases |
| Hydrolysis reactions | Hydrolases |
| Group transfer reactions | Transferases |
| Transfer of groups within molecules to form isomeric forms | Isomerases |
| Formation of double bonds by removal of groups | Lyases |
| Condensation reactions coupled to ATP cleavage | Ligases |
- Enzymes can enhance the rate of a reaction by up to 1020 folds.
Ex:
- Catalase.
Decomposition of hydrogen peroxide into water and oxygen.
One molecule of catalase can break 40 million molecules of hydrogen peroxide each second.
2. Carbonic anhydrase.
Catalyze the reaction of formation of carbonic acid inside the red blood cells.
It enables red blood cells to transport carbon dioxide from tissues to lungs. One molecule of carbonic anhydrase can process one million molecules of carbon dioxide each second.
3. Acetylcholinesterase.
Catalyze the breakdown of the neurotransmitter acetylcholine at several types of synapses as well as the neuromuscular junctions.One molecule of acetylcholinesterase can breakdown 25000 molecules of acetylcholine each second.This speed makes possible the rapid resetting of the synapse for transmission of another nerve impulse.
Enzymes catalyze reactions at body temperature when these reactions normally require high activation energy almost require over 1000C temperature for these reactions to occur. They do not change the equilibrium or the standard free energy change of reactions, there for enzymes are necessary for life. Otherwise reactions occur too slower rate for a metabolizing organism.
How enzymes catalyze reactions.
[g_article_ads]
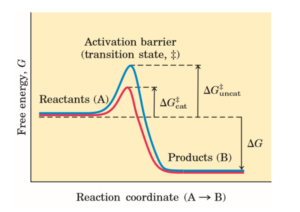
The rate of a reaction depends on the activation energy. If the activation energy is high, the rate of reaction is low. The reaction rate can be increased by providing favorable conditions like high temperature. A catalyst can provide a different path with a lower activation energy resulting an increase in the rate of the reaction.
Activation energy is the energy required to start a reaction.
energy changes during a chemical reaction in the presence and the absence of a catalyst.
How does an enzyme lower the activation energy of a reaction?
Mechanism of second order chemical reactions.
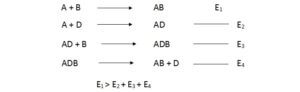
In second order reactions two molecules are react to form a third molecule.
For this reaction to occur,
Two molecules have to be closer enough so that their valence electrons can move between two sets of orbits.
When A approaches B, two set of electrons repel each other. Therefor A and B molecules have to collide violently to overcome the electron cloud repulsion.
Faster the molecules move, the greatest kinetic energy can be obtained.When kinetic energy is high, molecules can overcome electron repulsion and collide with each other.Motion of molecules can speed up by heating.
The activation energy of a specific reaction cannot be altered. But the mechanism of the reaction can be altered, hence the another set of reaction may be possible.
The activation energy of these two reactions may be lowered compared to first reaction. Therefore whole reaction can occur with low activation energy.
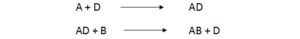
Substance such as D that permits a reaction to occur with low activation energy is called as catalyst. Catalyst holds two molecules closer together overcoming electron repulsion and allow valence electron to move between two orbits.
Ex:
Mechanism of first order chemical reactions.
[g_article_ads]
In first order reactions, only one molecule is involved and breakdown into two or more molecules. There is no molecular collisions occur there. Hence activation energy is required to vibrate the molecules to initiate the breakdown process.
When a molecule is heated up, it vibrate more rapidly initiating the breakdown of the molecule. Both first order and second order reactions can be accelerated by catalysts.
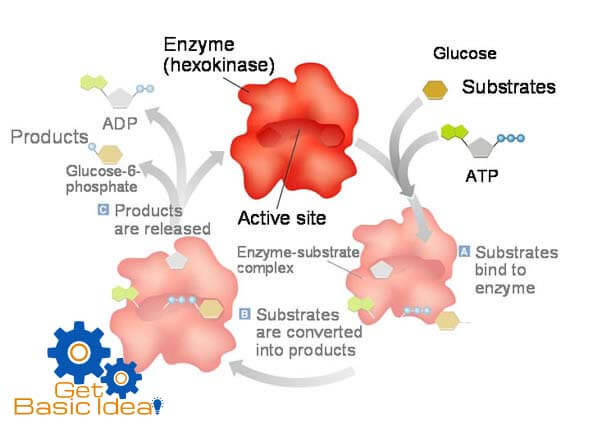
In any type of enzyme allows reactants to fit into a specific place called active site of the enzyme.
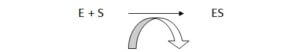
A simple enzymatic reaction can be written as follows.
E = enzyme, S = substrate, ES = enzyme- substrate complex, ES* = enzyme transition state complex, EP = enzyme product complex, P = product.
Active sites of the enzymes.
The active site of an enzyme is the specific place to bind the substrate. It has two parts.
- Binding site.
The place where substrate actually binds to the enzyme.
- Catalytic site.
The place where the reaction takes place.
Active sites have substrate specificity, means they can discriminate among possible substrates. Active sites are cable of recognizing specific functional group. Some active sites recognize only one type of molecule. They are capable of discriminating certain isomers. These are small tridimensional regions in the enzyme. Substrates binds to active sites through weak non covalent interactions such as hydrogen bonds, hydrophobic interactions and ionic interactions. These interactions hold substrates together in a proper orientation for effective catalysis.
Energy derived from bonding interactions, binding energy is used to reduce the activation energy of the reaction stabilizing the transition state complex.
Binding energy can be used as activation energy.
There are two models to describe enzyme substrate interactions.
- Lock and key model:
Substrate act as a key while enzyme act as a lock. Hence active site has a perfect shape which fits the substrate.
- Induced fit model:
This model states that the shape of the active site is flexible and can change complementary to the substrate.
Admin of Get Basic Idea / Senior Solution Architect.